Pesticide Registration Through Court Decisions Continue in Brazil
Just in the first three months of this year, there were 21 approvals through court decisions released by Anvisa”
Guest Author: Flavio Hirata, partner at AllierBrasil, a pesticide registration consulting firm. Email: flavio.hirata@uol.com.br

15 April 2024, Brazil: The new pesticide law in Brazil (Law No. 14785) implemented from 27th December 2023 has established deadlines for the conclusion of product registration evaluations. The time for registration approval is still very long as per the new law. According to the approvals of toxicological evaluations by Anvisa, published in the Federal Official Gazette of Brazil in 2024, the time to complete evaluations varies from 5 to 8 years in the cases of Formulated Products based on Equivalent Technical Products (generic products).
Deadlines for conclusion of registration claims and their amendments
| Registration type | Deadline for the conclusion of the evaluation |
| New Product – Formulated | 24 months |
| New Product – Technical | 24 months |
| Formulated Product | 12 months |
| Generic Product | 12 months |
| Identical Formulated Product | 60 days |
| Equivalent Technical Product | 12 months |
| Atypical Product | 12 months |
| Temporary Special Registration (RET) | 30 days |
| Product for Organic Farming | 12 months |
| Product Based on Biological Control Agent | 12 months |
| Pre-Mixture | 12 months |
Also, to speed up access to the market, companies continue to resort to court decisions against Federal agencies. Just in the first three months of this year, there were 21 toxicological evaluation approvals through court decisions released by Anvisa.
Approval of acts of toxicological evaluation of pesticides in compliance with court decisions (2024)
| Registrant | Brand name | Product | Date of Resolution by Anvisa |
| PERTERRA | EREDITÀ | Trifloxystrobin + prothioconazole | 03/27/2024 – Published in Federal Official Gazette on 04/01/2024 |
| PERTERRA | ATRAZINA 900 WG PERTERRA | Atrazine 900 g/kg WG | 03/22/2024 – Published in Federal Official Gazette on 03/25/2024 |
| ADAMA | BALTIC | Amicarbazone + isoxaflutole | 03/14/2024 – Published in Federal Official Gazette on 03/18/2024 |
| SYNCROM | RIDGE 88 SG | Glufosinate – ammonium salt 880 g/kg SG | 03/01/2024 – Published in Federal Official Gazette on 03/04/2024 |
| PERTERRA | LUFENURON 50 EC PERTERRA | Lufenuron 50 g/L EC | 02/29/2024 – Published in Federal Official Gazette on 03/04/2024 |
| XINGFA E WENDA | GLIFA 720 WG | Glyphosate 720 g/kg WG | 02/29/2024 – Published in Federal Official Gazette on 03/04/2024 |
| RAINBOW | COMPRAY FULL | Clomazone 500 g/L EC | 02/29/2024 – Published in Federal Official Gazette on 03/04/2024 |
| RAINBOW | POLESTAR XTRAL | Trifloxystrobin 490 + cyproconazole 210 g/kg WG | 02/29/2024 – Published in Federal Official Gazette on 03/04/2024 |
| RAINBOW | ACEWAY | Acetamiprid + bifenthrin | 02/22/2024 – Published in Federal Official Gazette on 02/26/2024 |
| ADAMA | CRONNOS ULTRA OD | Mancozeb +picoxystrobin + tebuconazole OD | 02/22/2024 – Published in Federal Official Gazette on 02/26/2024 |
| CROPCHEM | TACUAR 240 EC | Clodinafop-propargyl 240 g/L EC | 02/22/2024 – Published in Federal Official Gazette on 02/26/2024 |
| ADAMA | ALLURE BR | Clomazone + diuron + hexazinone | 02/08/2024 – Published in Federal Official Gazette on 02/14/2024 |
| CROPCHEM | CLODINAFOPE 240 EC CROPCHEM II | Clodinafop 240 g/L EC | 02/08/2024 – Published in Federal Official Gazette on 02/14/2024 |
| TRADECORP | CARIMBO 360 CS | Clomazone 360 g/L CS | 02/08/2024 – Published in Federal Official Gazette on 02/14/2024 |
| SYNCROM | DOMADO 88 SG | Glufosinate – ammonium salt 880 g/kg SG | 02/01/2024 – Published in Federal Official Gazette on 02/05/2024 |
| RAINBOW | ETHROLE | Ethiprole 200 g/L SL | 02/01/2024 – Published in Federal Official Gazette on 02/05/2024 |
| PROREGISTROS | GLUFOS-WYN 880 | Glufosinate – ammonium salt 880 g/kg | 02/01/2024 – Published in Federal Official Gazette on 02/05/2024 |
| RAINBOW | ATESALOR XTRA | Amicarbazone 700 g/kg WG | 02/01/2024 – Published in Federal Official Gazette on 02/05/2024 |
| CROPCHEM | DHARMA 100 SL | Cyproconazole 100 g/L SL | 02/01/2024 – Published in Federal Official Gazette on 02/05/2024 |
| SOLUS | ACEFATO SOLUS 970 SG | Acephate 970 g/kg SG | 01/29/2024 – Published in Federal Official Gazette on 01/30/2024 |
| ALAMOS | GLUFOSINATO 200 SL ALAMOS | Glufosinate 200 g/L SL | 01/03/2024 – Published in Federal Official Gazette on 01/08/2024 |
Out of the total approvals, 66% of the approvals of evaluations by Anvisa through legal action are from 4 companies, that is Rainbow with 24%; and Adama, Perterra and Cropchem with 14% each.
Approvals by Anvisa through court decisions (2024)
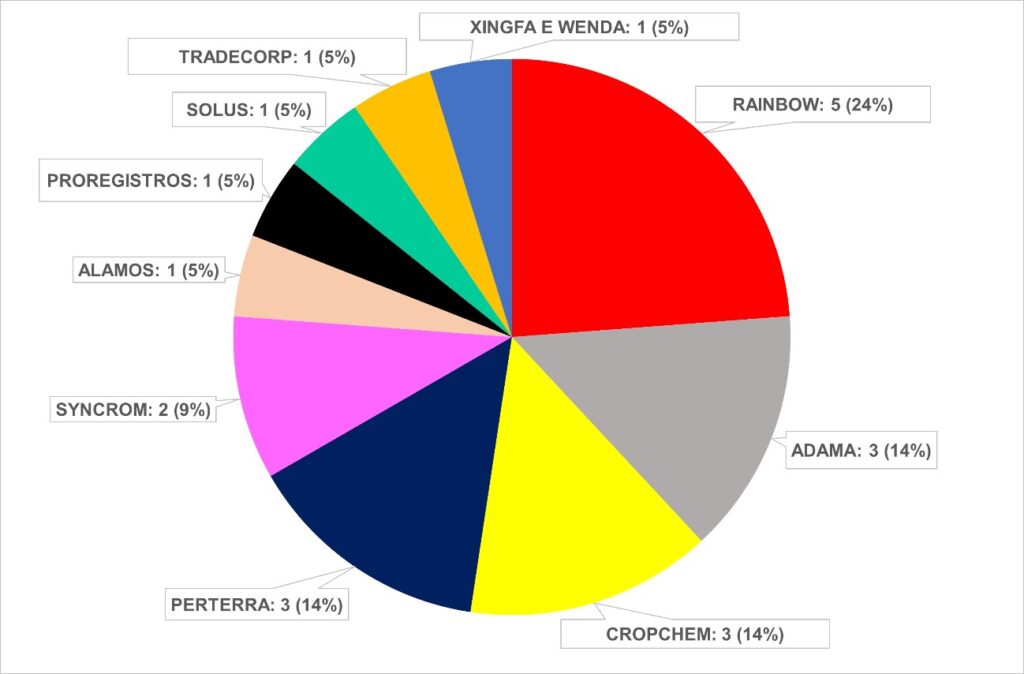
Source: Anvisa, adapted by AllierBrasil
According to a study regarding the registration requests approved through legal action in 2022, prepared by AllierBrasil and Mazza & Manente de Almeida Advogados, five Courts were responsible for 50% of the injunction approvals. In 2024, until 27th March 2024, one out of eight courts accounted for 33.33% of the 21 approvals.
Expectations are high regarding the new pesticide law and the deadlines for completing the evaluations of the registration processes. In the last two years, a significant number of companies, mainly from China and India have shown interest in accessing the Brazilian market.
Also Read: The Dominance of Lok-1: Madhya Pradesh’s Leading Wheat Variety
(For Latest Agriculture News & Updates, follow Krishak Jagat on Google News)